
MinerAlert

MinerAlert
Kelly Hall
2101 Sun Bowl Drive
500 W University Ave (general address)
El Paso, TX 79968
pave@utep.edu
The PAVE Unit conducts post approval evaluations, digital reviews, exit interviews, and on-site visits of active research protocols to corroborate alignment, compliance, and identify areas of improvement.
Who Conducts Evaluations: Trained PAVE staff members conduct post approval verification activities.
How Evaluations Are Initiated and Selected:How Evaluations Are Conducted: PAVE staff members review study documents and procedures, visit the research site, meet with the Principal Investigator, and provide feedback based on audit findings.
Reporting: Final Reports are provided within 7-10 business days after the exit interview. All reports are shared with PIs and pertaining oversight committees.
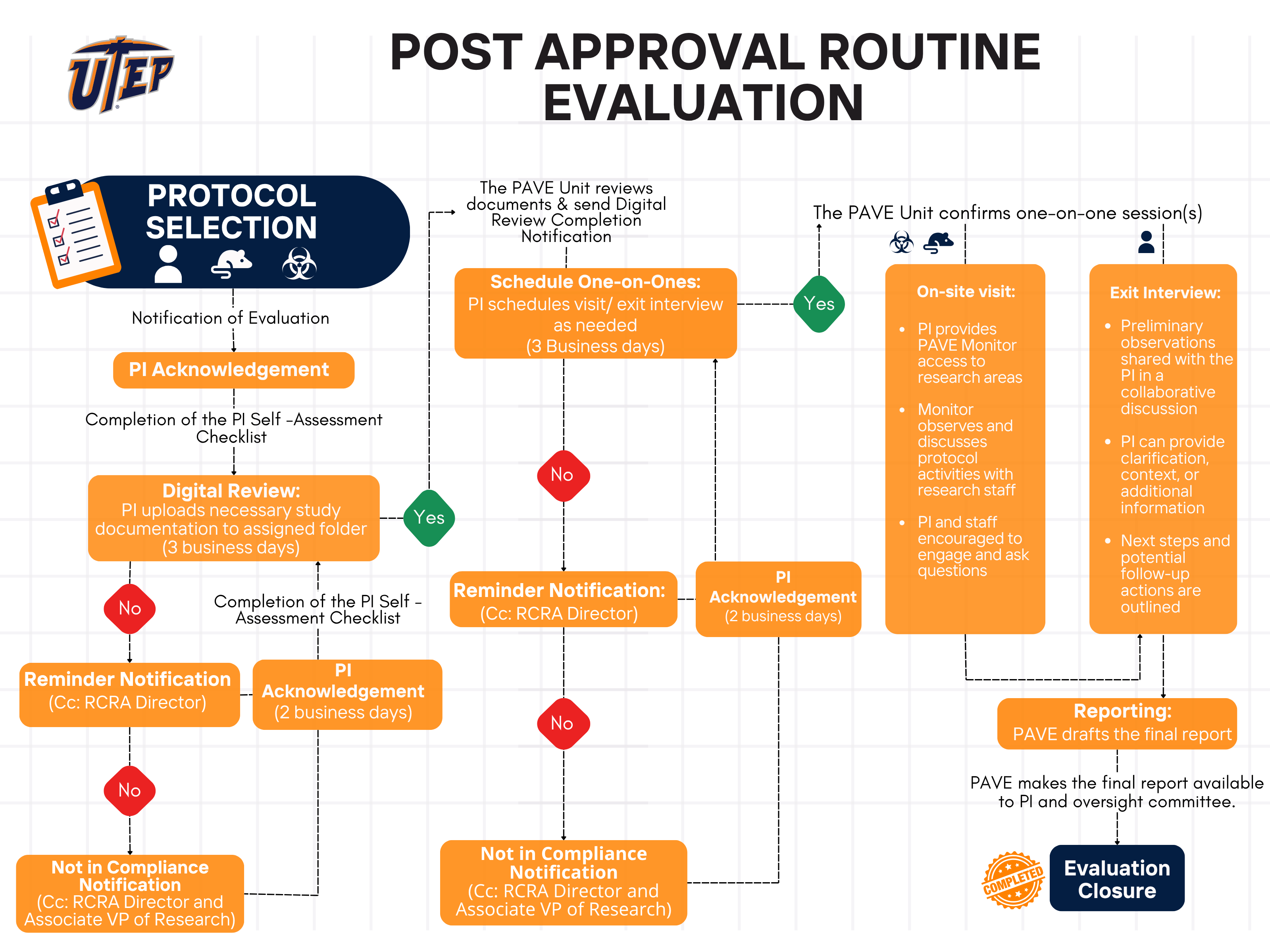
The following flowchart outlines the process involved in a routine PAVE evaluation. This process is designed to be supportive and educational, with follow-up support, training, and recommendations tailored to the specific needs of each study.
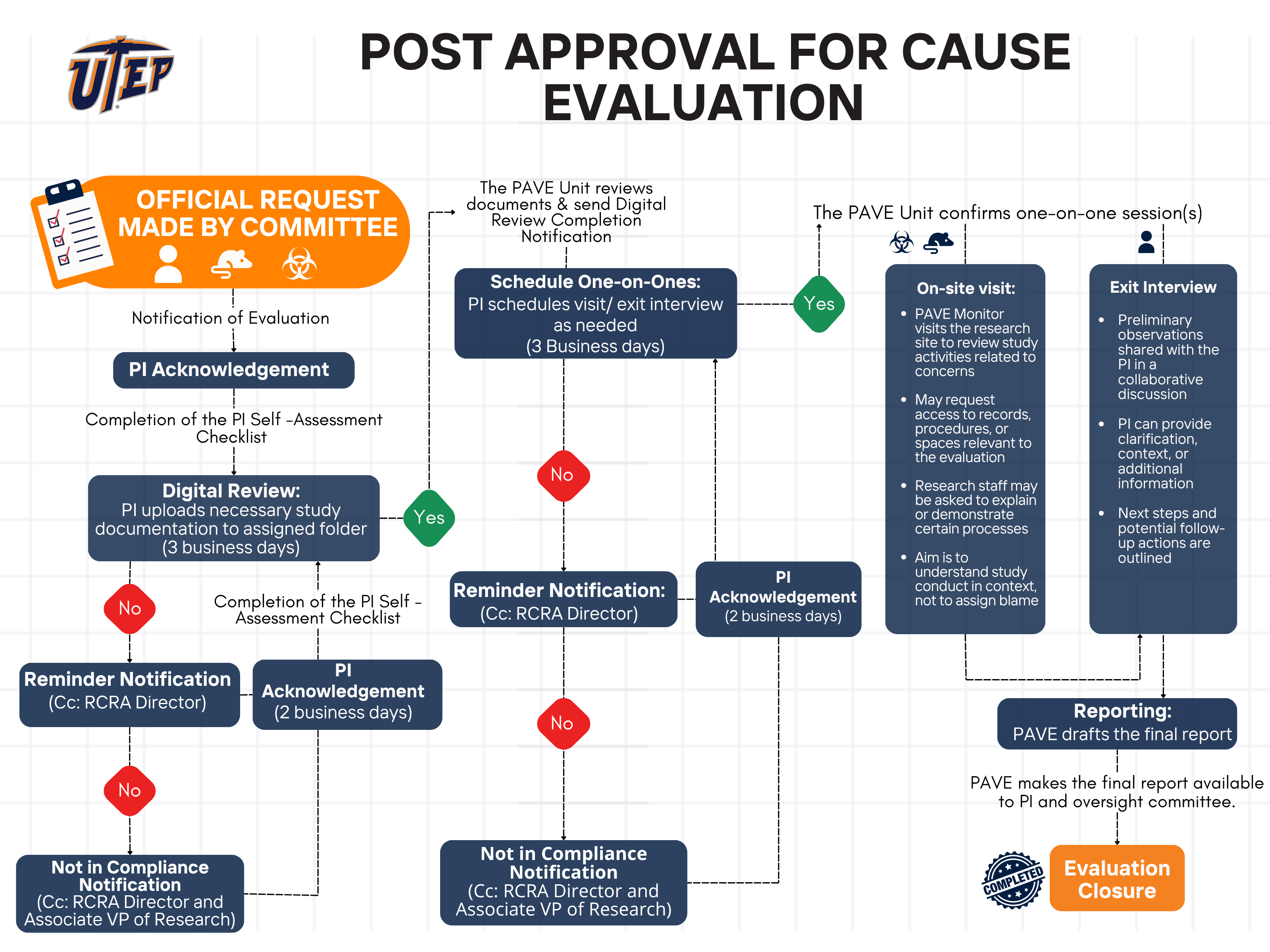
In the case of for-cause evaluations, the following flowchart illustrates the process initiated in response to specific concerns or reports. This evaluation is designed to address issues constructively, providing tailored recommendations and support to resolve identified challenges.
Upon completion of evaluations, reporting packages are shared with the relevant oversight committees (IACUC, IRB, IBC). These committees hold full authority to review findings, make classifications, recommend actions, and issue regulatory decisions. Outcomes vary based on results and committee review.
Possible outcomes include:
*Such outcomes are rare and are applied only in instances of serious non-compliance.